About the project
PIBD – Crohn’s desease (CD) and ulcerative colitis (UC)
The incidence, particularly of paediatric onset Inflammatory Bowel Diseases (PIBD, Crohn’s disease (CD) and ulcerative colitis (UC)) has risen dramatically in recent decades, with a marked increased incidence in regions where the disease used to be rare, such as South and Eastern European countries.
Compared to adult forms, paediatric onset of IBD reflects a more severe, potentially devastating life-long disease, usually requiring treatment with immunosuppressive drugs, and thereby exposing children to an increased risk of major side effects such as infections, new immune-mediated diseases and malignancies, in addition to the significant morbidity that accompanies these diseases.
A significant research gap at present involves identification of strategies to maximise therapeutic effectiveness without increasing risks from the treatment.
What is the aim of the project?
The major goal of this proposal is to develop and validate for the first time a treatment algorithm for children with PIBD based on predictors for high or low risk for early complicated or relapsing disease, improving effectiveness, while reducing treatment related risks and life-long complications due to uncontrolled disease progression (risk-stratified individualized treatment algorithm).
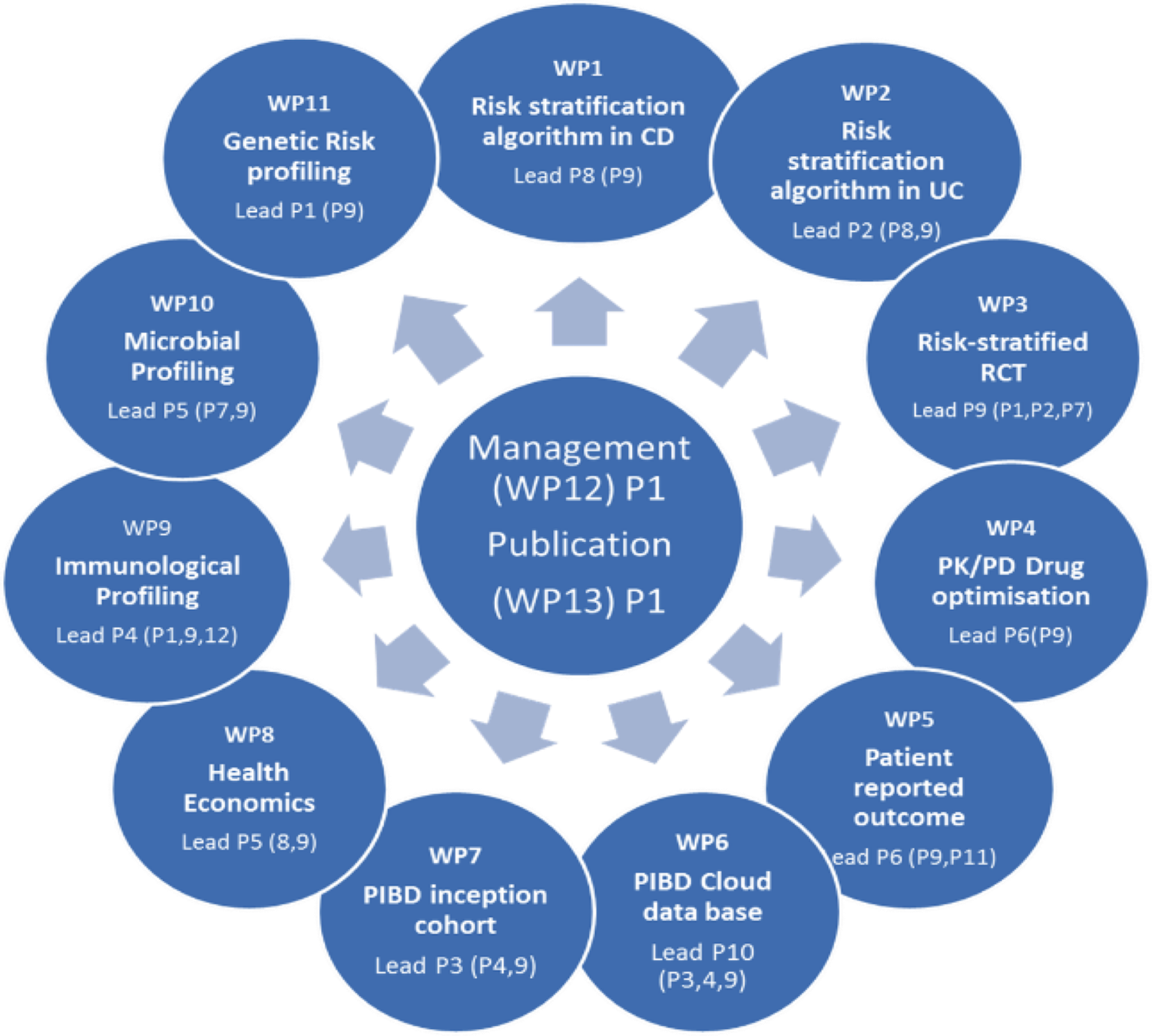
Three approcches
To attain this goal three approaches are combined based on an international network, PIBD-net (grouping all major PIBD centers in Europe and selected major sites from North Amercia, Australia and Japan):
-
Development of an accessible and feasible risk-stratified treatment algorithm for new onset paediatric IBD (based on an existing inception cohort) and validation of this algorithm in a new independent inception cohort.
-
Generation of a homogenized high-powered and secured data base to collect and analyse longterm real world data of children with IBD (inception cohort) in a prospective registry designed to analyze effectiveness and safety signals and correlate them to individual risk factors (genetic, immunological and microbiological).
-
Design and performance of a risk algorithm-based prospective large-scale multicenter randomized clinical trial (RCT). For the first time a RCT will be based on a stratification of patients into high versus low risk groups for early complicated/relapsing disease based on predictors at presentation in order to provide optimal therapy (further optimized by drug monitoring), and using different treatment strategies in each arm.